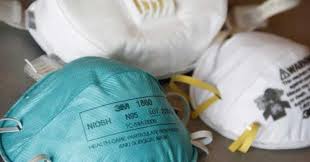
Lebanon VA’s Sterile Processing Service (SPS) implemented the new procedure this week which uses Steris V-PromaX to sterilize the protective masks for front line caregivers of patients who have or are suspected to have the coronavirus. The process uses vaporized hydrogen peroxide for disinfection. The SPS employees incorporated additional technology and monitoring into the process to improve safety beyond the federally mandated requirements. The SPS team also developed a system to ensure the safe return of N95 masks to the original user.
Lebanon VA Medical Center will utilize this revolutionary process to sterilize N95 masks for other medical centers within the Veterans Integrated Service Network (VISN) 4 as well as its own personnel. Current estimates are 500 masks will be sterilized each week. Medical Center director, Robert W. Callahan, Jr. stated, “While our local supply chain remains strong as we assist other facilities in our network, this innovation builds further depth in a crisis supply level of N95 masks if local supplies become challenged. I am extremely proud of our employees, their dedication to mission and their positive work ethic during this pandemic.”
This policy is activated only during emergencies, pandemics, or crises as identified by the Centers for Disease Control and Prevention (CDC) and supported by the FDA.